Abstract: The lumen maintenance rate of metal halide lamps is one of the important technical indicators of metal halide lamps. With the increasing demand for metal halide lamps in China and the international market and the continuous improvement of technology, metal halides The luminous flux maintenance factor of the lamp is more important. This paper focuses on the in-depth analysis of the mechanism and practice. Keywords: metal halide lamp, luminous flux, lumen maintenance, enthalpy loss, electrode. Analysis of the maintenance rate of a metal halide Metal halide lamps filled with different series of metal halides, different powers and different design structures have different luminous flux maintenance curves, such as most metal halide lamps in the early stage of lamp ignition (hours to one hundred hours) The luminous flux drops faster, and then the burning of the luminous flux is relatively stable. However, there are also some metal halide lamps whose luminous flux maintenance rate change curve is different. The rate of decrease of luminous flux at the initial stage of the ignition point is almost the same as the rate of decrease of the luminous flux at the later stage. The above differences are mainly due to the fact that the reasons for the decrease in luminous flux at the beginning and the end of the ignition point are similar but different. In order to deeply analyze the cause of the decrease of luminous flux in the ignition point of metal halide lamps, it is necessary to separately analyze the mechanism of light decay at the initial stage of the lamp and at the end of the ignition point in order to effectively improve the luminous flux maintenance rate of the lamp. Firstly, the mechanism of the decrease of luminous flux at the initial stage of ignition is analyzed. For example, the arc tube of a certain metal halide lamp includes: the size and shape of the quartz bulb and the electrode; the extension length of the electrode; the temperature of the cold end (including the size of the thermal insulation coating and Coating thickness); after the parameters and dosage of the filled metal halide pellets, and the input arc power and other parameters are determined, the change of the light flux is basically determined by: 1. The change of the light transmittance of the quartz bulb. 2. Changes in electrode emission properties (including cathode potential drop). 3. Changes in atomic concentration and atomic distribution of luminescent elements (Na, Sc, Dy, Hg---, etc.) in arc lamps of metal halide lamps. Since the total atomic radiation intensity in an arc tube of a metal halide lamp depends on the atomic concentration of the excited state, its expression is as follows: N k =N 0 (g k /g o )exp-(eV k /kT) Where N 0 is the atomic concentration of various luminescent elements. V k is the excitation potential of various luminescent elements. T is the temperature at which the atoms of each element are located. Since the metal halide lamp has a large temperature difference at different points in the arc tube at the ignition point, Figure 1 is an isotherm diagram of the 35W automotive metal halide lamp arc tube. Figure 1 shows the temperature profile of the plasma of a 35W automotive metal halide lamp. The electrode distance is 4.2 mm and the isotherm spacing is 250K. It can be seen from the above formula that an equal number of luminescent element atoms have different luminescence intensities in different isotherm regions. The molecular concentration of metal halides such as NaI and ScI3 under saturated vapor pressure is the surface temperature of the liquid metal halide adhered to the quartz tube wall near the cold end temperature of the arc tube (depending on the metal halide filling amount). The shape and state of the cold end surface and the velocity of the gas flowing through the liquid metal halide surface. It can be seen that the cold end condition of the arc will largely affect the atomic concentration and its distribution state, which will of course affect the luminous intensity of the metal halide lamp. Careful observation of the distribution of liquid metal halides near the cold end of the metal halide lamp in the ignition point is not difficult to find. The distribution of liquid metal halides near the cold end varies greatly from a few hours to several tens of hours in the initial stage of the metal halide lamp. (especially the Sc-Na series metal halide lamp), so the atomic concentration distribution in the arc tube changes greatly, which is one of the main causes of the initial light decay of the metal halide lamp. The second reason is the initial loss of é’ª(é•). For example, when the ignition point of the Sc-Na series metal halide lamp is used, the ScI 3 -NaI vapor pressure in the arc tube can be determined by NaScI 4 -NaI from the perspective of local chemical thermodynamic equilibrium (LTE). The molecular weight of NaScI 4 g in the molten salt is determined. I.e. to reduce losses Sc NaScI 4 moles, of course, cause ScI 3, the vapor pressure drop of NaI turned to the light emitting intensity decreases, the metal halide lamp ignition ScI initial concentration of 3 molecules of a filler metal halide of scandium, sodium It is determined by the temperature of the cold junction, but the ScI 3 and NaI molecules undergo a continuous gasification-liquefaction cycle during the ignition process, in which the ScI 3 molecules with higher gasification temperature are deposited on the bottom layer of the liquid metal halide, and then ScI 3 The molecules are also gradually deposited in the quartz crevice at the bottom of the electrode rod and at the groove of the quartz tube sealing portion, thereby causing a decrease in the bismuth and sodium ratio of the halide on the liquid surface, and reducing the NaScI 4 -NaI molten salt. NaScI 4 grams molecular weight, this stepwise deposition process will continue from fast to slow for a long time (hours to tens of hours). In addition, the chemical reaction between the crucible and the quartz tube wall of the tube also causes a loss of enthalpy. The chemical reaction formula is as follows: ScI3+SiO2+Hg=>Sc2Si2O7+HgI2 APL's Robert et al. used microscopic RAMAN detection to confirm that Sc2Si2O7 was generated at the beginning of the ignition point and caused helium loss. It is another major reason for the rapid decline in luminous flux at the initial stage of the ignition of Sc-Na series metal halide lamps. This process of luminous flux reduction can be clearly seen in the spectral test. Figure 2 shows the spectral energy distribution curve of the Sc-Na lamp. In order to compare the change of è¾å°„ è¾å°„ 强度 我们 我们 我们 我们 我们 我们 我们 我们 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 510 A description of the reduction of gaseous NaScI4 complexing molecules (deuterium loss). We used a set of 3 metal halide lamps to ignite 100, 500, and 1000 hours, respectively, and measured the average value of their relative energy changes at 510 nm and 580 nm. Table 1 and Figure 3 show the changes in the intensity of mercury and strontium lines during the ignition period. Corresponds to changes in lumen maintenance. If you need to read the full text of the study, please contact the China Lighting Network Special Information section editor: liuli# lightingchina.com.cn, when you send an email, change # to @,020-85521566-5273. Spot Lights Moving Head ,Led Moving Head Spot,Moving Head Spotlight,Spot Moving Head Light Guangzhou Cheng Wen Photoelectric Technology Co., Ltd. , https://www.cwledwall.com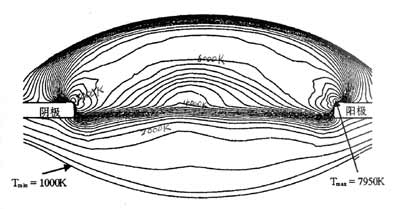
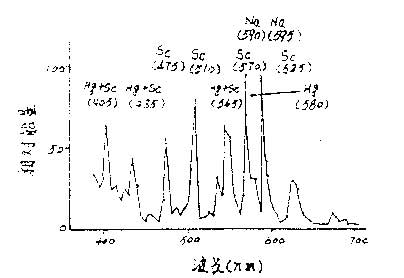
Fig. 2 Spectral energy distribution curve of Sc-Na metal halide lamp