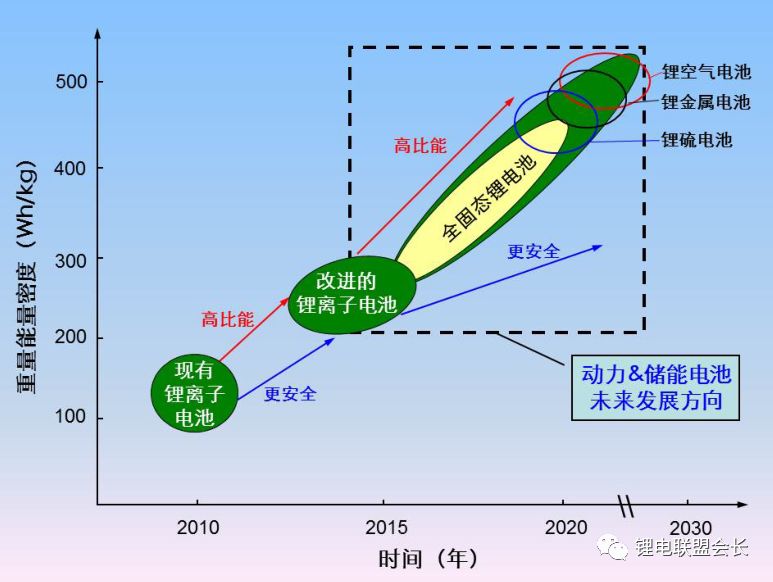
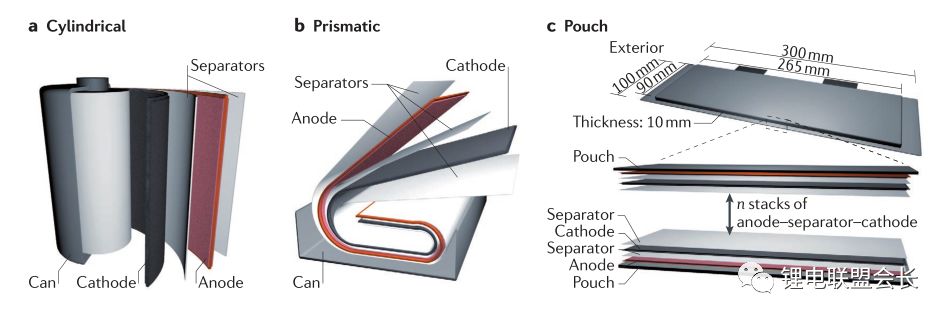
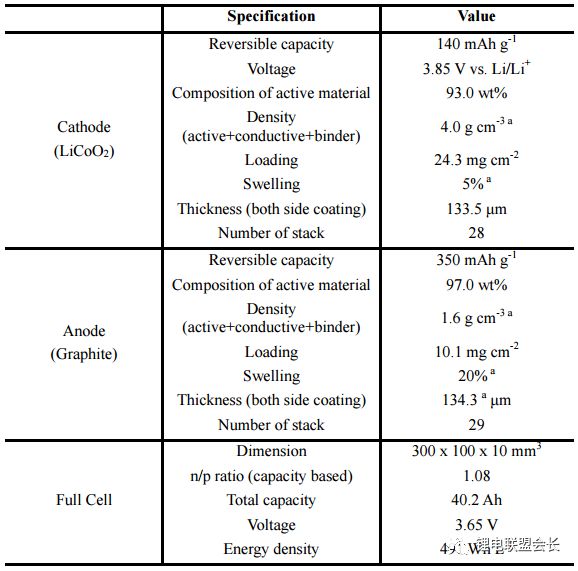
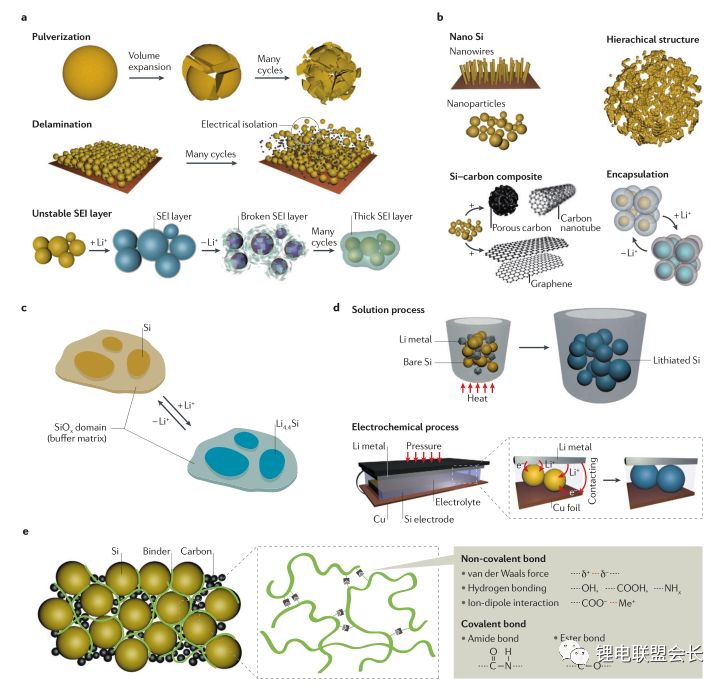
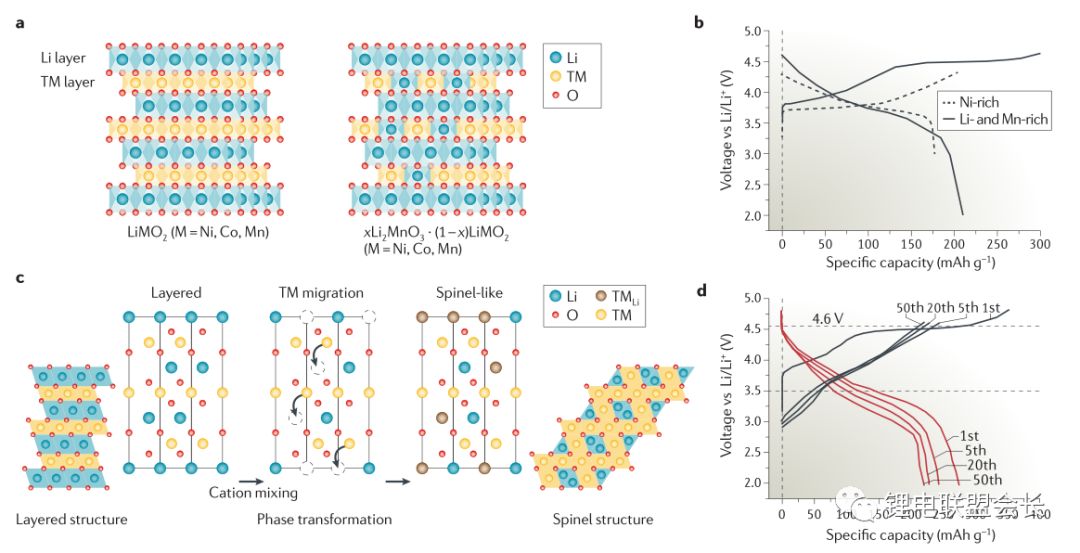
In the past decades, rechargeable battery technology has made continuous progress, and energy density is one of its main performance indicators. Now, lithium-ion batteries have surpassed lead-acid batteries, nickel-metal hydride batteries, etc., becoming a highly competitive commercial battery, but the performance of lithium-ion batteries still needs to be greatly improved, thereby extending the working time of mobile electronic equipment, and electric The mileage of the car, etc. This article will provide an overview of post-lithium materials and systems, discuss the working principles and problems of each post-lithium technology, and evaluate certain battery materials. Here we will divide the battery system into short-term and long-term technical solutions. The general indicators for evaluating rechargeable batteries include rate performance, cost, cycle life, and operating temperature range, but the increase in energy density has driven advances in battery technology over the past 150 years, such as lead-acid batteries (1850s) and nickel-cadmium batteries (1890s). , Ni-MH batteries (1960s) and lithium-ion batteries (1991-). Increasing the power consumption of mobile electronic devices and increasing the mileage of electric vehicles all require higher energy density lithium-ion batteries. The growth of the global electric vehicle market is slower than expected five years ago, reflecting the serious challenges faced by the battery industry. . The battery energy density mainly depends on the specific capacity and working voltage of the positive and negative electrode materials. Therefore, battery active materials have been the main research focus in the industry in recent years. The technical progress of the other components of the battery is getting smaller and smaller, including the separator, the binder, the conductive agent, the shell, and the components of the electrolyte. Embedded cell materials generally have fewer sites for lithium ion implantation and thus have limited energy density. In other words, if we want to significantly increase the energy density of the battery, we must go beyond the traditional embedding reaction mechanism and constantly explore new electrochemical reaction systems. Therefore, alloying, conversion reaction type electrode materials or gas phase reaction materials have attracted attention because their energy density may exceed that of the embedded type materials. These new electrochemical systems for energy storage are called post-lithium-ion batteries. The theoretical energy density of the rear lithium-ion battery system is higher, but its short life is also one of its serious problems. The main technical challenge is to overcome its poor reversibility. The poor reversibility of the post-lithium battery system is mainly due to the unstable phase transition of the active material and the uncontrolled side reactions occurring at the electrode/electrolyte interface. Therefore, it is necessary to continuously optimize the electrode structure and the electrolyte composition. Commercial battery configuration: In large-scale applications, a certain number of cells are assembled into one module. The design of the module depends to a large extent on the size and shape of the product and the internal connection circuit, safety requirements, temperature control, etc. . There are currently three types of commercially available batteries: cylindrical, square, and soft pack batteries. In a commercial lithium-ion battery, the typical 18650 cylindrical battery has a volumetric energy density of approximately 600–650 Wh/L, which is 20% higher than the volumetric energy density of similar square and soft pack batteries because of the cylindrical battery. Assembly stacks are more encrypted. In many practical applications, volumetric energy density is more important because most battery packs are designed according to a limited volume. Although the cylindrical battery has a higher volumetric energy density, the application of the square and soft pack battery is also extensive because of its high design freedom. Therefore, the relevant volumetric energy density values ​​below are based on the calculation of a long-thickness-thickness 300*100*10mm soft-cell battery in which n negative-separator-positive electrode layers are stacked. In this soft-pack battery, the volumetric energy density of the conventional LiCoO2-graphene battery is 491Wh/L, which is equivalent to the volumetric energy density level of many commercial batteries. Short-term technical solution: In the early stages of the post-lithium era, active electrode materials have been developed to a level that can be partially used in current products, and continuous research on these active materials will continue to increase their content in the electrodes. Silicon Negative Electrodes: Natural graphite and artificial graphite have long been used as the main active materials for lithium negative electrodes and have also been widely used as a reference for evaluating other emerging negative electrode materials. The silicon negative electrode has been widely studied because of its high theoretical capacity (4200 mAh/g) and suitable working potential (about 0.3 V, for lithium potential), which has been considered as one of the most promising negative electrode materials. As early as the 1970s, Argonne National Laboratories and General Motors Corporation did a lot of research on the negative electrode of silicon, aiming to overcome the large volume expansion (>300%) of the cycle of lithiation of the battery causing the degradation of the cycle performance. . By improving the structure of the electrode and the binder design, the problem of pulverization of the active material and detachment of the current collector is simultaneously solved, thereby improving the cycle performance of the battery. Another thorny problem with silicon anodes is that it is difficult to form a stable layer of SEI on the surface and therefore cannot provide effective protection on the surface of the electrode. This interface problem can be partially solved by selecting the appropriate electrolyte. From the perspective of the electrode structure, the synthesis of conductive nano-mesoporous composites through mesoporous carbon, graphene, carbon nanotubes, etc. can effectively alleviate the problem of volume expansion of the silicon negative electrode and significantly improve the cycle performance. The use of SiOx (x≈1) as a silicon-based negative electrode material is the first in the industry because the material can be produced on a large scale through gas-phase and liquid-phase reactions, and the quality is reliable and the price is reasonable (about 100$/kg). While graphene is 10-20$/kg). However, SiOx is usually mixed with graphene, and its content is generally less than 5%, reflecting the fact that the silicon anode application technology is not yet mature. The main problem of SiOx is that the first Coulomb efficiency is low. If the surface is not covered with carbon, the first reservoir is generally only 50-60%. The low coulombic efficiency results in the need for an excessively loaded positive electrode material, thereby reducing the overall battery energy density. Designing an active silicon structure requires both a long cycle life and a high first-time Coulomb efficiency, but these two parameters are mutually inconsistent. The buffer matrix and the mesoporous structure for long cycle life to relieve the volume expansion of the silicon negative electrode are not conducive to the first Coulombic efficiency. Improve because of excessive irreversible lithium ion consumption and interface reactions. In this case, pre-lithiation of the silicon-containing negative electrode material by a solution or an electrochemical process is an effective means to solve the first-bank problem without impairing the cycle life. Polymer binders are also important for improving the cycle performance of silicon anodes. Traditional PVDF is replaced by new binders, such as cross-linked polymers, self-healing polymer matrices, carbohydrate polymers and electronically conductive polymers. These new binders can effectively maintain the electrode structure through three-dimensional interchain interactions in repeated volumetric changes in the silicon anode. The basic requirements for the new binder research are: First, to maintain the new binder function at a lower binder content, and now the binder content in the industry is usually less than 5%; Second, the development of mixed polymer sticky The binding agent can simultaneously form an effective bond with silicon and graphene. Finally, the function of the binder is optimized so that the adhesion to silicon is enhanced, and the limitation of lithium ions is weakened, thereby facilitating the improvement of the Coulomb efficiency. In addition, supramolecular chemistry through the formation of hydrogen bonds, ionic bonds, and π-π interactions is also expected to increase the adhesion between the polymer and the active particles. Based on the size of the soft battery in the figure above, with a high-capacity LiNi0.8Co0.1Mn0.1O2 positive electrode and a graphene: SiOx=1:1 silicon-carbon composite negative electrode, the expected energy density will increase by 7.6% from 513 Wh. The /L (graphene) is increased to 552 Wh/L (silicon-carbon composite) when the volume of the negative electrode of the composite is approximately 110%. If graphene: pre-lithiated SiOx=1:1 is used as the negative electrode, the corresponding energy density can be increased to 628 Wh/L, because the positive electrode loading capacity will decrease after pre-lithiation, resulting in positive and negative electrode capacities. balance. If the volume expansion of the pre-lithiated SiOx and graphene composite negative electrode is only 50%, the overall battery energy density will be further increased to 710 Wh/L. This energy density value shows that achieving high first-time coulombic efficiency and suppressing electrode expansion is critical for obtaining a high-volume energy density battery using the high capacity characteristics of the silicon negative electrode. Layered nickel-rich, lithium-based, manganese-rich cathode materials: In the field of lithium-ion batteries, the next generation of emerging cathode materials is nickel-rich layered materials. Their origins can be traced back to the early research work of the Dahn and Thackere group. The other two common lamellar materials that have existed for a long time are LiCoO2 (~145 mAh/g) and LiNi1/3Co1/3Mn1/3O2 (~153 mAh/g). In fact, nickel-rich materials have already begun commercialization, LiNi0.8Co0.15Al0.05O2 is a typical representative. In addition, the introduction of manganese on the basis of this layered transition metal oxide facilitates the improvement of safety and rate performance, so a layered high-nickel ternary cathode material LiNixMnyCozO2 (x + y + z = 1) was developed in succession. x>0.6. All of these layered positive electrode materials have a common main body frame structure in which lithium and a transition metal are alternately arranged alternately in a closely packed cubic frame of oxygen atoms. Compared to conventional layered materials, nickel-rich materials have higher specific capacities due to favorable electronic structures, such as LiNiO2. In lithium- and manganese-rich materials, the increase in capacity is due to the presence of Li2MnO3 (in addition to the active LiMO2 lamellar phase), Li2MnO3 is activated during the first charge of lithium and the reduction of oxygen. The activation process occurring at a voltage greater than 4.7 V can provide a specific capacity of greater than 250 mAh/g for lithium-based and manganese-rich layered cathode materials. Despite having higher capacity, the two layered materials often face the problem of rapid capacity fade during the cycle, which is mainly due to the unstable structure and surface state changes. During the charging process, the two layered materials tend to transform into a thermodynamically more stable spinel phase after lithium ions are released. Crystallographically speaking, this spontaneous transition is caused by the lithium-transition metal misalignment of the octahedral sites in the lithium atomic layer by the transition metal, resulting in a drop in the voltage curve and capacity during discharge. This structural instability is also caused by an unstable interface, and transition metals from layered nickel-rich, lithium-based, manganese-rich cathode materials are prone to dissolution, resulting in irreversible and inactive interfacial compounds. In addition, these nucleophilic cathode materials react with substances in the electrolyte (eg, HF, PF5, etc.) to form a surface film and increase the electrode impedance. Then, through the transition metal doping and surface coating of AlF3, Al2O3, AlPO4, TiO2 and carbon, the formation of a spinel phase and a structure with a concentration gradient on the surface can effectively overcome the failure mechanism. However, the capacity decay due to the thermodynamic phase transition is difficult to make progress from the viewpoint of improving the electrolyte. The actual matching electrolytes of these two types of layered materials are basically based on cost considerations. Using graphene as the negative electrode active material, LiNi0.8Co0.1Mn0.1O2 or Li1.19Mn0.54Ni0.13Co0.12Ru0.01O2 replaces the conventional lithium cobalt oxide LiCoO2 as the positive electrode material, and the volume energy density of the battery is correspondingly from 491 Wh/L. Increased to 513 and 524 Wh/L (4.4% and 6.7% respectively). The increase in volumetric energy density is less than expected based on the calculated value of grams capacity because of its lower tap density (3.0–3.3 g/cm 3 ), and that of lithium cobaltate LiCoO 2 is 4.0 g/cm 3 . This also pointed out a more practical R&D direction for nickel-rich, lithium-based, and manganese-rich cathode materials: increasing the density of active powders and electrode sheets. If the negative electrode of silicon-carbon composite with graphene: SiOx=1:1, the corresponding volumetric energy density increases to 552 and 661 Wh/L, respectively. To sum up, the short-term goal for high-energy-density lithium-ion batteries is to adopt a silicon carbon composite material for the negative electrode and a high-nickel material for the positive electrode. This is exactly the current research stage that the industry is rushing to carry out. jobs. Gaming Earbuds With Mic,Metal Body Earphones,Sport Metal Headphone,Wired Metal Earphone Guangzhou YISON Electron Technology Co., Limited , https://www.yisonearphone.com